Optimizing Clinical Trials with CTIS: A Digital Transformation Blueprint for Pharma and CROs

The Clinical Trials Information System (CTIS), initiated by the European Medicines Agency (EMA), marks a significant transformation in the management and oversight of clinical trials across Europe. As clinical trials evolve, there is a critical need to streamline processes, enhance transparency, and ensure patient safety. The CTIS is designed to address these challenges, making it indispensable for pharmaceutical companies (pharma) and Contract Research Organizations (CROs).
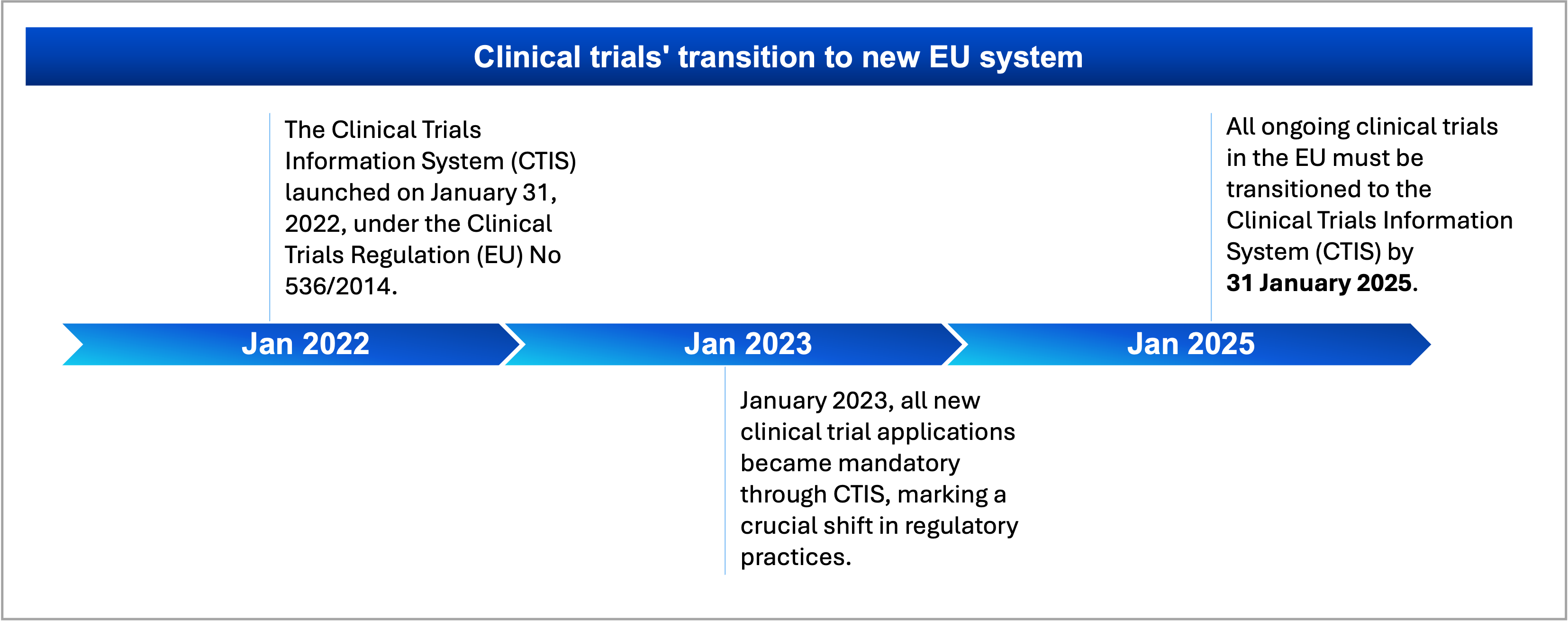
The Clinical Trials Information System (CTIS) launched on January 31, 2022, as part of the EU’s efforts to streamline clinical trial management under the Clinical Trials Regulation (EU) No 536/2014. Initially, its use was voluntary, allowing stakeholders to adapt. However, from January 2023, all new clinical trial applications became mandatory through CTIS, marking a crucial shift in regulatory practices. This transition period, lasting until 2025, aims to enhance efficiency, transparency, and collaboration in clinical research. Ongoing training and user feedback are vital to refining the platform, ensuring it effectively meets the needs of all participants in the trial process.
Also Read: Digital Immunity Explained: Minimize Downtime and Boost System Reliability
In today’s fast-paced healthcare environment, aligning with the objectives of the CTIS is key to ensuring that drug development is both efficient and compliant. Digital transformation will play a pivotal role in achieving these goals, and organizations that embrace modern technologies are more likely to thrive in this new landscape. This article explores the importance of CTIS and how pharma and CROs can maximize its benefits through tailored digital solutions.
The Need for CTIS in Modern Clinical Trials
1. Streamlining Regulatory Processes
One of the greatest challenges in clinical trials is navigating complex regulatory landscapes. Variability in submission and approval processes across different countries creates bottlenecks that can delay timelines. The CTIS simplifies this by providing a unified digital system that integrates regulatory workflows across multiple regions. This harmonization reduces the time, resources, and confusion involved in managing various regulatory requirements, ensuring faster approval and trial progression.
Also Read: Unleashing the Power of Azure AutoGen and AI Bots
2. Enhancing Transparency
Transparency is essential to building trust among stakeholders—regulatory bodies, researchers, healthcare providers, and patients. The CTIS provides real-time access to trial data, enabling better oversight and fostering accountability. This visibility not only elevates standards in clinical research but also ensures that participants and regulators are kept informed throughout the trial’s lifecycle.
3. Improving Patient Safety
A critical aspect of clinical trials is patient safety, and CTIS enhances this by integrating safety monitoring features. Real-time reporting of adverse events allows for quick interventions, protecting participants while improving trial quality. The ability to closely monitor patient data ensures that clinical trials maintain a high standard of care and ethical responsibility.
4. Fostering Collaboration
As the global healthcare market continues to expand, collaboration between international regulatory bodies becomes more vital. The CTIS enables seamless communication and data sharing, which in turn supports more efficient trial designs. By breaking down silos and promoting cross-border cooperation, the system helps ensure that trials meet the stringent standards of multiple regulatory agencies without unnecessary delays.
5. Meeting Market Demands for Speed and Efficiency
In an industry driven by the urgency to bring new therapies to market, the CTIS aligns with the demand for faster drug development processes. It allows for accelerated timelines without compromising patient safety or data integrity. This operational efficiency is critical as pharma and CROs face increasing pressure to deliver innovative treatments quickly, while adhering to stringent regulatory guidelines.
Also Read: Empowering Discovery: The Role of RAG Architecture & Generative AI in Healthcare & Life Sciences
Leveraging Digital Solutions to Maximize the Impact of CTIS
To harness the full potential of CTIS, pharma and CROs must adopt digital solutions that integrate seamlessly with the system’s framework. Here are key strategies that can help organizations stay ahead:
1. Clinical Trial Management Systems (CTMS)
Implementing a robust Clinical Trial Management System (CTMS) is crucial for managing the entire lifecycle of a clinical trial. From trial planning to data collection and reporting, a well-designed CTMS simplifies workflows and ensures compliance with regulatory standards. Features like site management and subject tracking help streamline trial operations, while real-time data access supports better decision-making.
2. Patient Engagement Platforms
Engaging patients is fundamental to the success of any clinical trial. Modern digital platforms facilitate communication between trial participants and coordinators, helping to improve recruitment, retention, and overall patient experience. These platforms provide patients with updates, trial information, and easy access to communication channels, keeping them informed and involved throughout the process.
3. Advanced Data Analytics
Data is a powerful tool in clinical trials, and leveraging advanced analytics can unlock valuable insights. By implementing predictive analytics and machine learning, organizations can identify patterns in trial data, optimize resource allocation, and enhance patient recruitment strategies. This data-driven approach also supports faster decision-making, ultimately leading to more efficient trial designs and outcomes.
4. System Integration for Real-Time Reporting
Integrating existing systems with CTIS ensures that data flows seamlessly across platforms, enabling real-time reporting and monitoring. Effective data integration reduces redundancies, improves accuracy, and ensures that all stakeholders—from sponsors to regulatory bodies—have access to the most up-to-date information. This capability is vital for identifying potential safety issues early on and maintaining trial integrity.
5. Automation to Reduce Administrative Burden
Manual processes in clinical trials often result in delays and increased workloads for trial staff. Automating routine tasks such as data entry, monitoring, and reporting can free up valuable time, allowing staff to focus on more critical research activities. Automation also reduces the risk of human error, ensuring that data remains accurate and regulatory submissions are timely.
User-Centric CTIS Design for Better Adoption
The success of any new system hinges on user adoption. CTIS is no exception, and organizations must focus on creating intuitive user interfaces that are easy to navigate for all stakeholders, including researchers, patients, and regulatory bodies. Training programs tailored to each user group are also essential to maximize the system’s capabilities and ensure smooth adoption.
1. Intuitive Interfaces for Better User Experience
Designing user-friendly interfaces is critical to the successful adoption of CTIS. Whether used by researchers or patients, an intuitive system minimizes friction and increases satisfaction. By reducing the learning curve, organizations can improve system utilization and encourage collaboration.
2. Comprehensive Training and Support
To maximize the benefits of the CTIS, organizations must invest in comprehensive training and support. This ensures that users across all levels—researchers, clinicians, and regulatory authorities—are proficient in the system. Continuous support services can also address any challenges that arise during the system’s implementation, helping to ensure that transitions are smooth and disruption-free.
Ensuring Compliance and Mitigating Risk
Regulatory compliance is a non-negotiable aspect of clinical trials. The CTIS helps organizations stay aligned with evolving regulatory requirements, but proactive strategies are necessary to ensure ongoing compliance.
1. Staying Ahead of Regulatory Changes
As regulations continue to evolve, it is critical to stay informed. Regularly monitoring updates from agencies like the EMA helps ensure that systems and processes remain compliant. Having a proactive compliance strategy in place reduces the risk of costly delays due to non-compliance.
2. Audit-Readiness Through Documentation
Maintaining meticulous documentation is essential for regulatory audits. Comprehensive record-keeping, supported by digital systems, ensures that trials are transparent and can withstand scrutiny. This also fosters trust with regulatory authorities, creating a foundation for smoother audits.
Patient-Centricity at the Core of CTIS
Successful clinical trials hinge on patient engagement. A patient-centric approach—supported by digital platforms—ensures that participants feel informed, involved, and valued.
1. Real-Time Communication
By implementing communication channels that provide real-time updates to patients, trial sponsors can increase engagement and improve retention rates. Patients who feel empowered by having access to timely information are more likely to remain engaged throughout the trial.
2. Feedback Mechanisms for Continuous Improvement
Establishing channels for patient feedback creates a loop of continuous improvement. Patient experiences and concerns provide critical insights that can be used to refine trial processes, making future trials more effective and patient-friendly.
Conclusion
The CTIS represents a transformative shift in the clinical trial landscape. For pharmaceutical companies and CROs, the system provides opportunities to streamline operations, enhance transparency, and improve patient safety. Embracing digital transformation and integrating tailored solutions will enable these organizations to fully leverage the CTIS, driving efficiency, collaboration, and better outcomes in clinical trials.
By aligning with the CTIS framework and adopting modern digital strategies, pharma and CROs can accelerate their development timelines while ensuring compliance and safety. As the industry moves towards a more transparent and patient-focused future, the ability to innovate and adapt will be critical to success.
With a deep understanding of clinical trial processes and expertise in digital transformation, Apexon is uniquely positioned to support pharma and CROs in this evolving landscape. From advanced analytics to seamless integration with CTIS, our solutions are designed to enhance efficiency, patient engagement, and compliance. To learn more about how we can help your organization navigate this transition and unlock the full potential of CTIS, we invite you to connect with our team.